DEPECT
DEsign Protein and Enzyme by Computational Tools
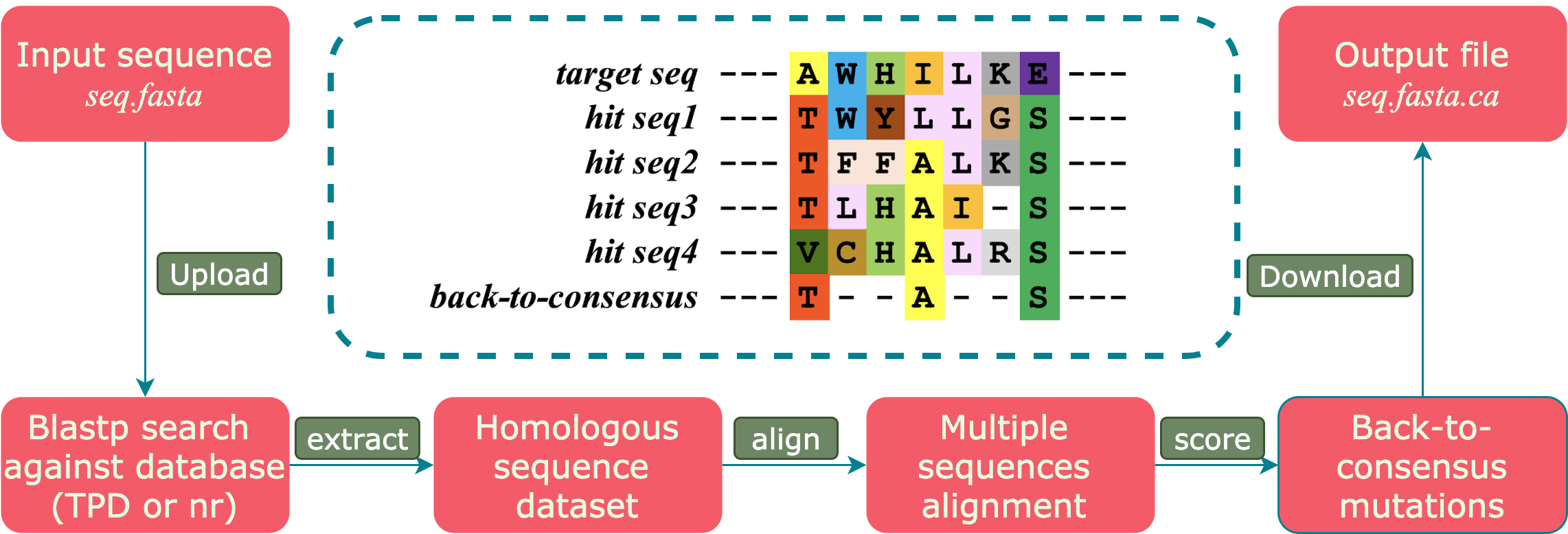

The server accepts accept only ONE AMINO ACID sequence as the target sequence. The back-to-consensus mutations suggested by TPD are likely to contain more information on how proteins naturally adapted the heat. Meanwhile, due to the limited species range, more genetic drifted mutations contributed by phylogeny are embedded. Comparing to the TPD, mutations suggested from the nr database will not contain too much phylogenetic bias. Nevertheless, those mutations only providing informations of a consensus sequence from a higher dimension which likely to tolerant all observed mutations.
Warning: Please do not click the submit button multiple times!
In the post-genomic era, genome mining has been one of the most powerful tools leading to discovery of new enzymes with properties of interest, and the combination of biological feature like thermophilic and the sequence-based method like BLAST is the dominant approach to conduct the mining. In this way, numerous of enzymes has been found and some of them have industrial applications.
However, as we can see, most of the naturally evolved enzymes need redesign to fit the relatively harsh industrial conditions. Directed evolution, the Nobel winning technique, enlighten us on the power of evolution again. With many successful examples using consensus analysis to improve thermostability, we believe that from the way thermophiles evolved to fit the high temperature we can learn how to redesign enzymes of interest to be more stable